Vaccine ready – are we there yet?
This is an edited version of the full article, which is available here. The article pre-dated the US Election.
Executive summary
Societies and governments around the world are looking at a COVID-19 vaccine as the panacea that will enable the world to recover, reconnect and regain our way of life. Since the genetic code for COVID-19 was unlocked, researchers have set out in an unprecedented and coordinated effort to make a vaccine available on a mass scale.
Today, there are more than 150 vaccines being developed around the world, with many of these in late-stage clinical trials. Governments, industry and philanthropists have injected tens of billions of dollars into the development of vaccines with the aim of accelerating a process that normally takes around 5 to 15 years. Timeframes for achieving a safe, clinically validated and commercially ready COVID-19 vaccine vary but there are several front-runners that aim to have initial doses available within the next several months.
Vaccines currently in Phase 3 trials
There are currently 11 vaccines in Phase 3 clinical trials. Of these 11 vaccines, two are in combined Phase 2/3 trials, four are in Phase 3 trials and the remaining five are approved for early or limited use while still in Phase 3 trials (four of which are in China and one in Russia). Unfortunately, there is limited publicly available information about the vaccines being developed in China.
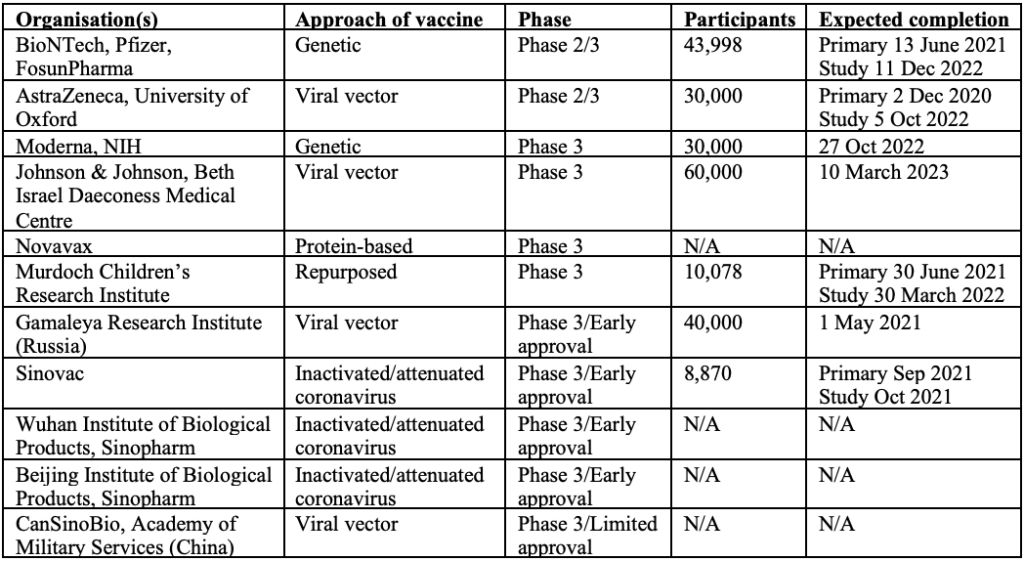
In order to get full immune response, most leading vaccine candidates will require two doses 28 days apart, other than Pfizer’s, where the second dose is administered 21 days after the first. The first shot would prime the immune system, helping it recognise the virus, and the second shot would strengthen the immune response.
Operation Warp Speed
Operation Warp Speed (OWS) is a partnership that includes a number of American organisations – the Food & Drug Administration (FDA), the National Institutes of Health (NIH), the Biomedical Advanced Research and Development Authority (BARDA) and the Department of Defense (DoD). The goal of OWS is to produce and deliver 300 million doses of vaccine, with initial doses available by January 2021. The Trump administration hopes that by providing additional funding to those drug manufacturers with leading vaccine candidates, these companies can significantly compress the development timeframe. To date, approximately US$10 billion worth of federal funding has been disbursed under OWS.
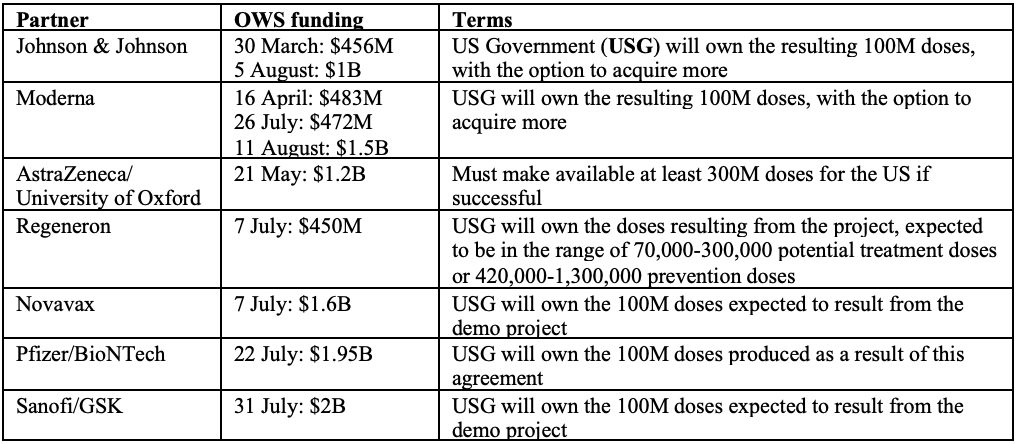
Note that the above table only includes funding designated to vaccine development and some (but not all) of the funding designated to manufacturing (where the recipients are Big Pharma). About $1.5 billion more has been allocated to other US manufacturers who are not Big Pharma, and to distributors.
There have been recent claims that the Trump administration has attempted to shape the FDA’s protocols and policies surrounding granting of emergency use authorisations (EUAs) to COVID-19 vaccines, with the goal of eliminating a provision that would effectively preclude a vaccine before Election Day. However, the FDA has stated that it has communicated its new standards directly to drug makers and has made it clear that its requirement for two months of safety data would remain, as would the requirement for an independent panel of experts to assess each vaccine candidate before a final decision is made.
In Europe, the European Medicines Agency (EMA) is using “rolling” reviews to significantly fast-track the approval process. This involves assessing data on experimental candidates before all of the evidence needed to make a final decision is available. The EMA has already begun evaluating Pfizer and BioNTech’s candidate, with their Phase 3 trial, expected to recruit between 37,000-44,000 participants, having already administered the second dose of the vaccine to more than 28,000 as at the date of this article.
The Australian landscape
In August, the Australian government signed a letter of intent with AstraZeneca, under which the British pharmaceutical giant would provide 25 million doses of its vaccine, most likely manufactured at the CSL laboratories in Melbourne.
Upon signing of the AstraZeneca letter of intent, CSL commented that the development of its own vaccine, in collaboration with the University of Queensland (UQ), remained its immediate priority. Only a few weeks later, the Australian government signed another agreement with CSL, this time for the supply of 51 million doses of the UQ vaccine, should it prove successful.
The Australian government has promised citizens that, should either of these clinical trials prove successful, a vaccine would be made available to each Australian free of charge.
A third domestic candidate is being developed by the Murdoch Children’s Research Institute (MCRI). It is currently in a Phase 3 trial involving 10,078 participants. MCRI’s vaccine candidate is a repurposed tuberculosis vaccine, with completion of the study estimated for early 2022.
Conclusion
There are a lot of observations we can make about the battle against COVID-19. We saw Wall Street suffer its worst week since the GFC in March 2020, governments forced to make tough decisions and prioritise certain demographics over others, unprecedented travel restrictions and citizens largely confined to their homes for weeks or months at a time. We have seen the catastrophic impact that an unlucky string of genetic mutations can have on the world, but also the speed and co-ordination with which the world’s scientific community can spring into action to combat it. Never before have we possessed the knowledge and resources to facilitate such a flow of information, the analysis of so much data and, despite the restrictions on travel, the focusing of the world’s greatest minds on one common goal.
Above all, this pandemic has once again reinforced the importance of health. The desire for ourselves, our families, friends and neighbours to live healthy and long lives is one of a handful of traits shared by every community in the world. This is what pushed the global effort to combat COVID-19 into overdrive. Health will remain a priority long after the pandemic is over.
As the world looks to the health industry for a solution, capital markets have either recovered or are on their way to doing so. We have seen what happens when our health is in jeopardy, and learnt the costs of not being prepared. It is critical that we consider the lessons this pandemic has taught us against our core values and priorities and move swiftly to implement necessary changes. Once COVID-19 has been brought under control, the most important thing that governments, investors and individuals can do to avoid a repeat is to support the industry that we quite literally cannot live without – healthcare.
* * *
This article was provided by the Bio Capital Impact Fund (BCIF). BCIF is a global evergreen fund, investing into both public and private companies in the healthcare sector.